The Modern Atom (1950's - present)
Current Model of the Atom

- Many scientists contributed to our modern model of the atom.
- We no longer believe that electrons orbit the nucleus. In fact, in experiments done to identify the location of the electrons, we find that the best we can do is predict what probability one would have if looking for the electron in a particular location.
-
There are defined regions where you are likely to find electrons. These three dimensional volumes are called orbitals. Depicted above is an atom with a nucleus containing protons, and neutrons, surrounded by an electron "cloud". This cloud represents the space around the nucleus where you are likely to find its electrons. The lighter the color the less likely you are to find the electron there. This cloud is the orbital. Click on the images below to get a sense of the structure of an atom.

- Orbitals have several shapes. Each shape is correlated with a particular
energy level. This makes the
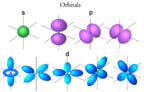 model consistent with the spectroscopic data that show electrons can only
exist in specific discrete energy levels. Click on the illustration to the
right to see cartoon images of these orbitals.
model consistent with the spectroscopic data that show electrons can only
exist in specific discrete energy levels. Click on the illustration to the
right to see cartoon images of these orbitals. - So, spectroscopy can be explained by the orbital shape. An electron of higher energy will most likely be found in an orbital that is different from the region that the electron will be found in when it is in a lower energy state. The electron cannot be in an in-between energy state or orbital.
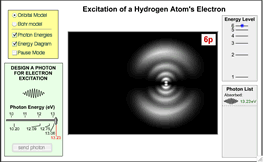
- See a Flash application that shows both an orbital model of an atom and Bohr model as it abosorbs and emits EMR.