Balancing Equations
- Remember Lavoisier's Law of Conservation of Mass? So far the chemical
equations we have written have not taken this law into consideration.
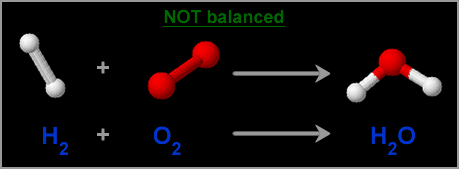
Where did the other oxygen atom go?
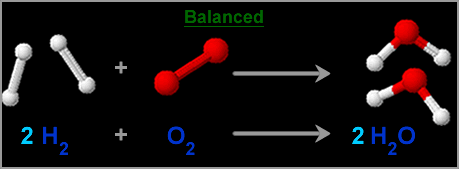
- If we use both oxygens, then we will need two more hydrogen atoms. If the equation is written like this then the Law of Conservation of Mass is maintained.
- Let's recall the reaction between Sodium Chloride and Lead(II) Nitrate:
NaCl(aq)
+ Pb(NO3)2(aq)
--> NaNO3(aq)
+ PbCl2(s)
Where did the second chloride ion come from, and where did the other nitrate
ion go?
- Every atom that appears on the left side of the arrow must also appear on
the right side. It might be tempting to fix this problem by rewriting PbCl2
as PbCl, but that would be the incorrect formula for Lead(II) Chloride. Pb+2
must pair up with two Cl-1s.
- We resolve this by placing coefficients in front of the formulas indicating
that you can have different ratios of the substances reacting to form different
ratios of products. To fix the above reaction we would rewrite it as:
2 NaCl(aq)
+ Pb(NO3)2(aq)
-->2 NaNO3(aq)
+ PbCl2(s)
- The 2 in front of NaCl give you 2 Nas and 2 Cls. The 2 in front of the
NaNO3
gives you 2 Na, 2 N, and 6 O. If you count up all the atoms on the left and
right of the arrows you will have the same number of each element. The equation
is now balanced.
- An unbalanced equation is like having a recipe with no quantities for each
ingredient.