Pure Substances vs. Mixtures
- Matter can be classified in to two broad categories: pure substances and mixtures.
- Pure substances
- Elements - all the same type of atom.
- elemental info
- The Elements Song by Tom Lehrer
- elemental info
- Compounds - substances made from two or more different kinds of atoms.
- Elements - all the same type of atom.
- Mixtures
- Homogeneous
- Mixtures which are the same throughout with identical properties everywhere in the mixture.
- Not easily separated.
- This type of mixture is called a solution. A good example would be sugar dissolved in water or some type of metal alloy like the CROmium-MOLYbdenum steel used in many bike frames.
- Heterogeneous
- Mixtures which have different properties when sampled from different areas.
- Examples of this would be sand mixed with water or peanuts mixed with raisins.
- Homogeneous
- Atoms vs. Molecules
- Atoms - the smallest piece of matter you can have that chemists can do reactions with is an atom. Each element has it's own type of atom. How to distinguish between atoms will be explained in a later unit.
- Molecules - two or more atoms bonded together with a covalent bond (more
on that bond later) is called a molecule.
- If all the atoms bonded together are of the same time the molecule formed is still an element.
- If different types of atoms are bonded together, then the molecule
formed is a compound.
a single atom
(of an element)a molecule
(of an element)a molecule
(of a compound)
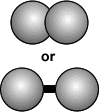
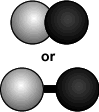
Note: Atoms don't have a color. The colors here are used to differentiate between kinds of atoms.
- Click the image below for a graphical representation of these ideas.
- Using molecular modeling kits create several examples of an element, compound, and a mixture.
Image from: Fred Senese at Frostberg State Univ.
