Fission and Fusion
- In addition to decaying, a nucleus can be transformed in two other important ways: fission and fusion. The isotope iron-55 is the most stable nucleus. Atoms with higher mass will tend to go through fission and give off energy while atoms with mass lower than iron-55 can go through fusion to become more stable and give off energy.
- Fission
- If an unstable nucleus breaks into two smaller pieces, it can form two new atoms. This is known as fission.
- When this occurs some energy is also released. This is the source of energy that powers nuclear power plants and the first nuclear bomb.
- See the illustration below:
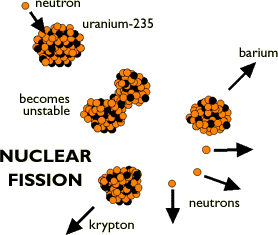
(Used with permission. Original found at: http://www.sciencenet.org.uk/database/Physics/Atomic/p00435c.html)
- The above illustration could be written symbolically as:
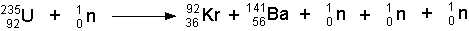
(note: The two nuclei that form can vary. They are not necessarily the isotopes listed above.)
- Check out an animation of this at: http://www.atomicarchive.com/Fission/Fission1.shtml
- Only a few isotopes spontaneously fission. Uranium - 235 and Plutonium - 239 are the ones most commonly used for this purpose.
- Fusion
- If two nuclei collide with enough force then they may fuse together forming a larger single nucleus. This is known as fusion.
- When this occurs some energy is also released. This is the source of energy that powers the sun (as well as other stars) and hydrogen bombs.
- See the illustration below:
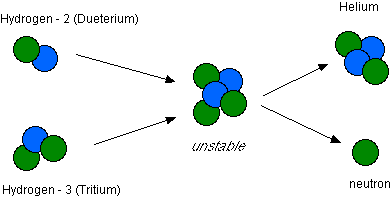
- The above illustration could be written symbolically as:
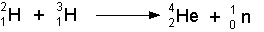
- Check out an animation of this at: http://www.atomicarchive.com/Fusion/Fusion1.shtml
- Energy Released
- Einstein's famous equation states that: E = mc2
E = energy
m = mass
c = speed of light =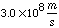
- This means that mass can be converted into pure energy. The amount of energy is determined by multiplying the mass times the speed of light squared.
- Consider the following table:
Mass of reactants: Mass of 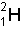
3.344548 x 10-27 kg Mass of 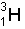
5.008347 x 10-27 kg Total -->8.352895 x 10-27 kg Mass of products: Mass of 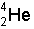
6.64658 x 10-27 kg Mass of 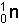
1.674954 x 10-27 kg Total -->8.321534 x 10-27 kg
Missing Mass = 8.352895 x 10-27 kg - 8.321534 x 10-27 kg = 0.031361 x 10-27 kg
- Somehow the products weigh less than the reactants! Einstein said that
this missing matter was converted to energy according to his famous equation:
E = mc2
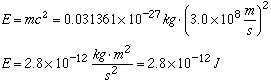
- That is a tiny amount of energy, but that is only for one reaction.
In 1 kg of starting material there could be 1.2 x 1026 nuclear
reactions.
1.2 x 1026 . 2.8 x 10-12 J = 6.7 x 1014 J of energy released
That is equivalent to the energy released in about 10 Million tons of TNT!
1 kilogram of nuclear fuel could theoretically yield as much energy as
10 million tons of TNT!
- As you can see, nuclear reactions can produce an enormous amount of energy. Both fission and fusion produce energy in this way. A bomb releases the energy all at once while a nuclear power plant releases the energy slowly over very long periods of time. Currently we don't have any way to control a fusion reaction. All nuclear power plants in operation today use fission to produce energy.
- Fusion reactions similar to the one described above are the source of power for the Sun and other stars. Notice how the lighter hydrogen nuclei fuse to form a heavier helium nucleus. Inside the sun heavier and heavier atoms are then fused together to form more and more elements. That is where all of the elements heavier than helium have come from - inside a sun. You are made from atoms that were once at the center of some star which exploded sending out all the elements it created through nuclear fusion in its core. You are made from old stars!
- Einstein's famous equation states that: E = mc2