Half-life
- Radioactive materials by their very nature, decay via various methods to form other elements, ultimately to form stable non-radioactive isotopes.
- If you monitor a radioactive substance with a Geiger counter, you will hear
"clicks" every time the device detects a particle of radiation.
Over time, if you graphed the number of clicks per second you would see a
graph resembling something like you see below:
- Depending on how quickly an element decays, the time needed to make the above graph could take anywhere from a fraction of a second to billions of years.
- To see a visual depiction of how this graph is formed go to: http://www.colorado.edu/physics/2000/isotopes/radioactive_decay3.html
- One way to describe how fast a radioactive substance decays is to determine
how long it will take half of the currently present atoms to decay. Let's
look at a particular example - Iodine-131 which is used to diagnose problems
with the thyroid gland.
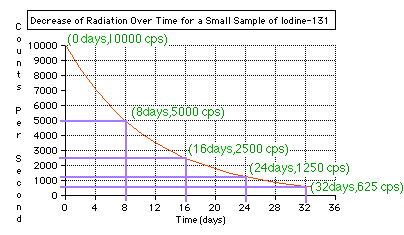
By looking at the graph you can see that half of the Iodine-131 has decayed every 8 days. We then say that Iodine-131 has a "half-life" of 8 days.
If you started with 80 grams of radioactive Iodine-131, how long would it take until you had less than 5 grams left?
- The shorter the half-life the more unstable the atoms of that isotope are,
and the faster they will decay.