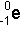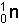Types of Radiation
- The term "radiation" is often used to refer to different
things. There are two main types of radiation: electromagnetic waves and high
energy particles. When people use the term "radiation" sometimes
they are refering to electromagnetic wave, sometimes high energy particles,
and sometimes both.
- Electromagnetic Radiation
- Electromagnetic Radiation is a type of energy that travels
in waves.
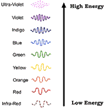
- Types of EMR

- Radio waves
- Microwaves
- Infrared
- Visible light
- Ultra Violet
- X-Rays
- Gamma Rays
- Handout: Electromagnetic
Waves
- To talk of a particular kind of energy we can discuss the energy, wavelength,
or frequency of the wave
 .
.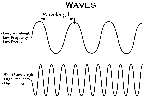
- Wavelength is the distance from the peak of one wave to
the peak of another.
- Frequency is the the number of waves per second that a stationary
observer would count while the wave is passing by.
- Energy, frequency, and wavelength are all interrelated due to the
fact that all EMR travels at the same speed. As wavelength decreases,
frequency increases, and energy increases
- Gamma rays have the highest frequency, shortest wavelength, and
highest energy. These are represented by the following symbol:
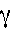
- Lower energy forms of EMR (radio, infrared, etc.) are not harmful.
- High Energy Particles
- Tiny subatomic particles can have high energy if they are moving
at very fast speeds.
- The most common forms of high energy particles are:
- Alpha Particles
- These are represented by the following symbols:
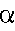 or
or 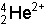
- As you can see from the symbol above an alpha particle is the
same as the nucleus of a Helium atom (or a Helium atom with no
electrons), a cluster of two protons and two neutrons.
- Beta Particles:
- These are represented by the following symbols:
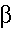 or
or 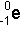
- As you can see from the symbol above a beta particle is the
same as an electron.
- Neutrons:
- These are represented by the symbols: n
or
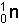
- All of the particles above get their high energy when they are ejected
from the nucleus of an unstable atom. That is why they are all the familiar
particles - protons, neutrons, and electrons - that make up atoms.
- Radioactivity
- Radioactive substances emit some or all of the particles above,
as well as, gamma rays, the highest energy form of the electromagnetic
waves. Other forms of electromagnetic radiation (x-rays, light, radio,
etc.) are not directly emitted by radioactive substances.
- Some radioactive substances emit primarily alpha, beta, or gamma. Most
emit several types of radiation as the nucleus decays and becomes more
stable.
- When and how this radiation is released will be discussed later.

 .
.
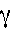
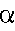 or
or 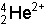
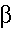 or
or