Dipole-Dipole and Hydrogen Bonds
- Some molecules form areas of positive and negative charge formed
through an uneven sharing of electrons (polar covalent bonding). Water is
formed with polar covalent bonds between hydrogen and oxygen. Below is water.
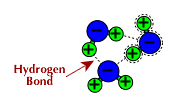
- Because part of the molecule is partially positive (not as positive as an ion with a +1 charge) there are attractions between the negative portion of one molecule and the positive portion of another molecule. This attraction forms weak bonds between molecules
- When hydrogen is one of the atoms within a molecule that is attracted to
the dipole on another molecule, this somewhat stronger dipole-dipole attraction
is called a hydrogen bond. The hydrogen bond is the attraction between
molecules, not the covalent bond which is formed between hydrogen and an atom
from its own molecule. Below are some examples of hydrogen bonding.
- Click on the image below to see hydrogen bonds represented as dotted lines in this computer model.
- To see a 3D view of water and it's hydrogen bonds in motion go to: http://cps.bu.edu/education/vmdl/software/water.html and follow the instructions for installing the software. (Windows only.)
- Hydrogen bonding is also an important factor in helping to shape the
structure of larger molecules. DNA is an excellent example.
- Click on the image below to see hydrogen bonds represented as dotted lines in this computer model.
- A molecule can have more than one polar region, so the more polar regions a molecule has, the greater two molecules of this kind will attract to each other.
| Name | Propane |
1-Propanol |
1,3-Propanediol |
| Boiling Point | -42°C |
97°C |
214°C |
| Structural Formula | 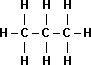 |
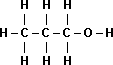 |
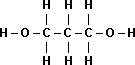 |
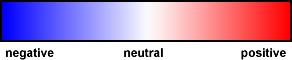
| Surface Charges |
|
||
 |
|||