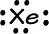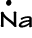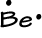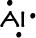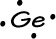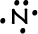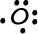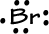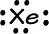Intro to Lewis Dot Notation
- Valence electrons are those electrons that are in the highest
principle energy level. It is these electrons that primarily interact
with other atoms.
- Oxygen has 6 valence electrons: 1s22s22p4
- Sodium has 1 valence electron: 1s22s22p63s1
- Bromine has 7 valence electrons: 1s22s22p63s23p64s23d104p5
- Xenon has 8 valence electrons: 1s22s22p63s23p64s23d104p65s24d105p6
- Pick two elements from any column and determine how many valence electrons
those atoms have. You should notice a particular pattern.
- Bonds form when valence electrons are shared or transferred from one atom
to another, so our focus will mainly be on these highest energy electrons.
- Lewis Dot diagrams are a graphical way of showing how many valence electrons
an atom has. Below are some examples of electron configurations and the Lewis
Dot diagram for each of those atoms.
- Sodium has 1 valence electron: 1s22s22p63s1
with the Lewis Dot Diagram ->
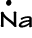
- Beryllium has 2 valence electrons: 1s22s2
-->
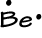
- Aluminum has 3 valence electrons: 1s22s22p63s23p1
-->
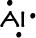
- Germanium has 4 valence electrons: 1s22s22p63s23p64s23d104p2
-->
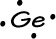
- Nitrogen has 5 valence electrons: 1s22s22p63s23p3
-->
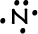
- Oxygen has 6 valence electrons: 1s22s22p4
-->
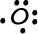
- Bromine has 7 valence electrons: 1s22s22p63s23p64s23d104p5
-->
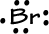
- Xenon has 8 valence electrons: 1s22s22p63s23p64s23d104p65s24d105p6
-->