Lewis Dot Structures - Understanding Molecular Structure
- To help determine how atoms will covalently bond together into molecules we can use Lewis Dot Diagrams.
- Lewis Dot Diagrams show only the valence electrons and utilize the fact that most molecular compounds have non-metal atoms sharing these electrons to have a stable 8 like the Noble Gases.
- Below are some common Lewis Dot Symbols for some atoms:
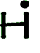




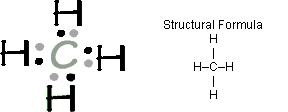
- Each unpaired dot represents a valence electron that can be shared - a place
where a bond can form. Methane with the formula CH4
is diagrammed as:
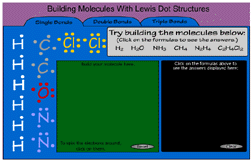
- Below is a flash application to help you practice making molecules. Try to make each of the molecules listed by dragging atoms to the green work area. If you need to rotate the electrons just click on them.