Periodic Properties and the Structure of the Table
- Elements in the same group (or column) have the same chemical properties.
- All the elements in a group (or column) are called families.
- Group 8: The Noble Gases, don't react with other elements.
- Group 1: The Alkali Earth Metals, all react with water in the following
manner
2 Li + H2O ---> H2 + 2 LiOH
2 Na + H2O ---> H2 + 2 NaOH
...
2 Fr + H2O ---> H2 + 2 FrOH
- Periodic Properties
- As you move across a row various properties change regularly
click on the images below to see a visualization of the various properties.
All of these images are from www.webelements.com,
one of the best periodic table sites on the web.
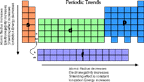
Periodic Trends 



Summary of Trends
- As you move across a row various properties change regularly
click on the images below to see a visualization of the various properties.
All of these images are from www.webelements.com,
one of the best periodic table sites on the web.
- Early on the elements were divided into two broad categories -> metals
and non-metals. This was done long before anyone knew any detail about the
atoms or any of the periodic properties mentioned above.
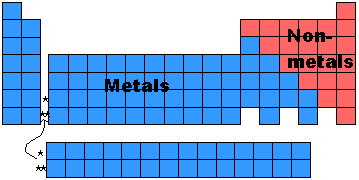
- As you can see what makes something a metal or a non-metal is based on other properties like ionization energy, atomic radius, and electronegativity
- When metal atoms are bonded together the electrons become delocalized, jumping
from one atom to another. A common analogy is to say that the nuclei of atoms
in a metal exist in a "sea of mobile electrons".
- This is due to the low ionization energy of these electrons, and is what
gives metals the property of conductivity. A typical electric current can
be described as electrons moving from one place to another. This can easily
happen in metallic substances as depicted below.
- Why does it make sense that atoms on the lower left of the table have the most metallic character and those on the upper right have the most non-metalic character. Look at the properties and how the trends change as you go across or down the table.