The Role of Charge in Bond Formation
- Valence electrons are the electrons which are in the highest principle energy level orbitals.
- These electrons are, on average, the furthest from the nucleus and the most free to interact with other atoms.
- Electrons are negative, and the nuclei of atoms are positive. Through the
interaction of electrostatic charge (+ and -) between atoms, bonds can be
formed.
Seeing the Effect of Positive Charge on Orbitals
An atom and its electrons: The electrons are attracted to the other positive charge: 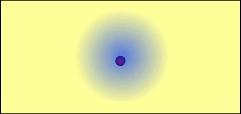

Starting with two separate atoms, the electrons are attracted to each nucleus: The attraction of the electrons for each nucleus is balanced by the repulsion of the two nuclei - a covalent bond is formed: 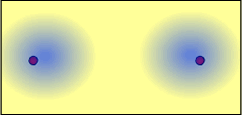
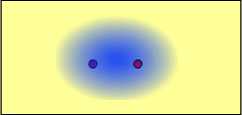
- The number of valence electrons will dictate the number of bonds that can form or the charge of an ion, and the electronegativity of the atoms will dictate the type of bond that will form.