Shapes of Molecules
- You may have noticed that sometimes there are multiple ways to
construct a molecule from the atoms given in the formula. Take C4H10
for example :
n-butane iso-butane (2-methypropane) - Each of the compounds displayed above is an isomer of butane. Isomers refer to different molecules with the same formula.
- Check out the Isomer Construction Set by Fred Senese at Frostburg State.
- Did you notice that the molecules shown above have a particular shape to them?
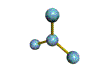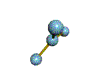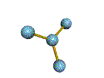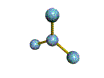
- Molecules will form into shapes such that regions of high electron density (where electrons are being shared between atoms and where there are unshared pairs of valence electrons on the surface).
- Because all of these regions are negatively charged, they repel each other and try to move as far away as possible from each other.
- Depending on how many atoms are bonded and if there are unshared pairs of electrons around, you will see the following common shapes:
|
|
|
|
|
|
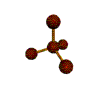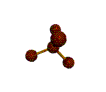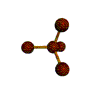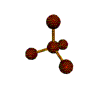
|
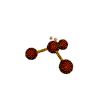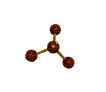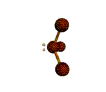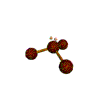
|
|
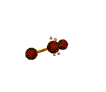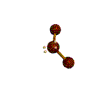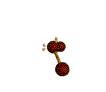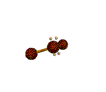
|

|
- Check out the images of molecules below and see where you can find these shapes within them. The images are being displayed by the Chime plug-in which allows you to manipulate and take measurements on the displayed molecules. See the Using Jmol sheet for instructions.
- Bring up a set of molecules that are associated with the Exploring Molecular Shapes guide.
- The shape of molecules is extremely important, especially for larger more
complex molecules. Below are several examples showing how shape is the key
factor in a molecules biological function.
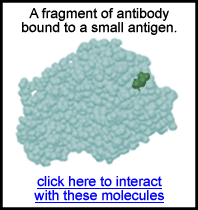
- Immune function: Our immune system has the capability to recognize
foreign material that enters our body. It does this not by "thinking"
about it. After the first time our immune system encounters a pathogen,
it makes antibodies that are just the right shape to bond with
the foreign antigen. Below is an example showing the antibody molecule
in blue and the antigen in green:
- Immune function: Our immune system has the capability to recognize
foreign material that enters our body. It does this not by "thinking"
about it. After the first time our immune system encounters a pathogen,
it makes antibodies that are just the right shape to bond with
the foreign antigen. Below is an example showing the antibody molecule
in blue and the antigen in green:
- The synthesis of ATP (the primary energy storing molecule in our body) happens in a series of steps using ATP synthase.
- Click here to see how some molecules can change shape in order to better fit with another molecule.