Valence Electrons
- Valence electrons are those electrons that are in the highest
principle energy level. It is these electrons that primarily interact
with other atoms.
- Oxygen has 6 valence electrons: 1s22s22p4
- Sodium has 1 valence electron: 1s22s22p63s1
- Bromine has 7 valence electrons: 1s22s22p63s23p64s23d104p5
- Xenon has 8 valence electrons: 1s22s22p63s23p64s23d104p65s24d105p6
- Pick two elements from any column and determine how many valence electrons those atoms have. You should notice a particular pattern.
- Experimental evidence shows us that atoms are most stable when they have full s and p orbitals (8 valence electrons) in their highest principle energy level.
- One simple piece of evidence for this is the Noble Gases which form the last column on the right of the periodic table. All of these elements have 8 valence electrons in their highest principle energy level. One exception is He which has a full principle energy level with 2 electrons.
- If atoms don't have 8 valence electrons (or two if they are close to He), then they will react with other atoms in order to have 8 valence electrons. This can be done by either sharing electrons between atoms, taking electrons from other atoms, or giving electrons away to other atoms.
- Basically, atoms are most stable when they can achieve an outer electron structure similar to the closest Noble gas.
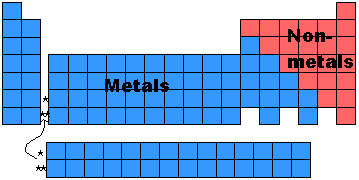
- The periodic table can be divided into two broad groups:
- Metals (low ionization energy and low electronegativity)
- Non-Metals (high ionization energy and high electronegativity)
- If two atoms are going to bond together we have three possible categories:
- metal/metal
- metal/non-metal
- nonmetal/nonmetal
- Kinds of bonds that can form
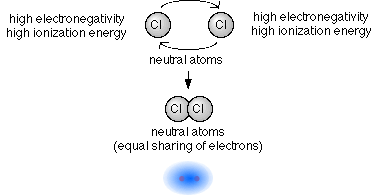
- If we have a metal/metal or a nonmetal/nonmetal pairing then each atom in the pair will have a similar electronegativity and ionization energy as the atom it is bonded with. In that situation the atoms will attract the other's electrons with about the same strength, and it will take a similar amount of energy to remove an electron from each atom.
- When both atoms in the pair have a similar pull on the other's electrons
(electronegativity) and resist the removal of an electron (ionization
energy) in a similar way, then they will share electrons forming a covalent
bond.
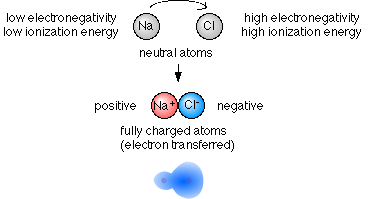
- If we have a meta/nonmetal pairing then the nonmetal atom will pull strongly on the metal's weakly held (low ionization energy) electron and the metal atom will not pull very strongly (low electronegativity) on the strongly held (high ionization energy) electrons of the nonmetal atom.
- When there is a large imbalance of electronegativity and ionization
energy between atoms, an ionic bond will form.
- The valence electrons will determine how many electrons are shared or transferred between atoms.