Catalysts
- A catalyst is a substance which speeds up the rate of reaction without being used up in the reaction. You will have the same amount of catalyst at the beginning and end of a reaction, but the reaction will occur much more quickly.
- Catalyst examples:
- Most of the enzymes in your body are catalysts. Many of the chemical reactions that are necessary for life to occur run too slowly without being catalyzed by an enzyme. Without catalysts we could not exist.
- The catalytic converter that is part of all modern car exhaust systems. This turns many of the pollutants (primarily hydrocarbon (C-H) fragments and carbon monoxide (CO) in the exhaust) into carbon dioxide (CO2) and water (H2O).
- When you generated oxygen from hydrogen peroxide earlier this year you
used a Manganese metal catalyst.
2 H2O2 ---> 2 H2O + O2
Notice that the MnO2 is not written as part of this reaction. That is because it is not consumed during the reaction. It can be reused over and over again. - Chlorine atoms from CFCs (chlorofluorocarbons) catalyze the breakdown of ozone into oxygen: O + O3 ---> 2 O2
- A catalyst works by lowering the activation energy necessary to complete
a reaction
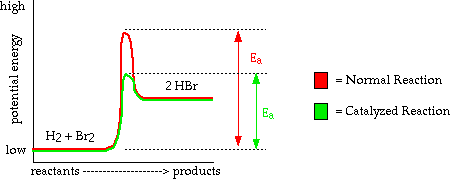
- Because it takes less energy to form products, the reaction occurs more quickly.
- Film: Catalysts and Activation Energy
- Film: Hydrogen Catalysis