Chemical Equilibrium
- Often we talk about reactions as if you mix a couple of things
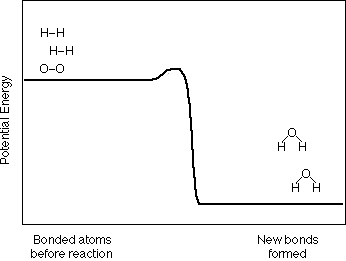
together to produce one or more new substances. For example, we say that Hydrogen
and Oxygen can combine to make Water and write the chemical equation as:
2 H2 + O2
 2 H2O
2 H2O
- Why don't we write the equation as: 2 H2 + O2
 2 H2O
2 H2O
In other words, why is it less likely that water breaks up to form hydrogen and oxygen than it is for hydrogen and oxygen to form water? - In fact, there is a very small chance that water could break up to form
hydrogen and oxygen. Maybe we should write the chemical equation as:
2 H2 + O2 2 H2O
2 H2O
to show that it is mostly water that is formed.
- However, so little water breaks up to form hydrogen and oxygen that it is
OK, and considered correct to write the equation as:
2 H2 + O2
 2 H2O
2 H2O
- However, now consider the following reaction in which the potential energy
of the reactants and the products are almost the same, making the activation
energy almost equal from left to right as it is from right to left:
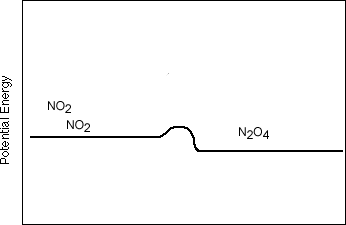
- If we start with all NO2 , then they will collide and start to form N2O4. At first the likelyhood of two N2O4 molecules colliding will be small, but eventually the N2O4 will build up in concentration until it will be common for them to collide. Because they can almost as easily break up into NO2 as NO2 can combine to form N2O4 the reaction will start to go to the left. Eventually, you will have just as much N2O4 forming as NO2 forming. In other words, the reaction will go left and right at the same rate, reaching an equilibrium.
- All reactions are equilibrium reactions. However, many (like the formation of water from hydrogen and oxygen) are much more likely to happen one direction that we think of these reactions as only going in that direction. For those reactions it is OK to use a single arrow.
- For reactions in which a significant amount of product and reactants are constantly being formed we use the double arrow to indicate the behavior of this equilibrium.