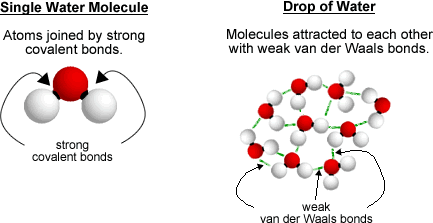
Strong and Weak Bond Overview
- There are two major bond types: weak bonds between moleucles, and
strong bonds between atoms.
- Weak bonds - van der Waals bonds
- All atoms and molecules have a weak attraction for each other. This
is called the van der Waals attraction
- All liquids and some solids are formed through this kind of weak bond.
- Hydrogen bonds which are commonly referred to in biology, which hold
our two strands of DNA together, and help shape proteins into their proper
conformation, fall into this category.
- Strong bonds - ionic and covalent
- When two atoms bond together very strongly they do so via either a covalent
bond or an ionic bond.
- These bonds are MUCH stronger than the weak van der Waals bonds that
help molecules to stick to each other.