Activation Energy
- Some reactions are spontaneous, some require energy to get started,
and some require continual input of energy to continue reacting. This can
be explained through the idea of potential energy in chemical bonds and a
concept called activation energy.
- Chemical bonds can be a way of storing energy. Energy is absorbed to break
bonds and energy is released upon the creation of bonds. If the amount of
energy absorbed in a reaction is greater than the amount of energy released,
the reaction is endothermic. If the amount of energy absorbed is less than
the amount of energy released then the reaction is exothermic. See diagrams
below:
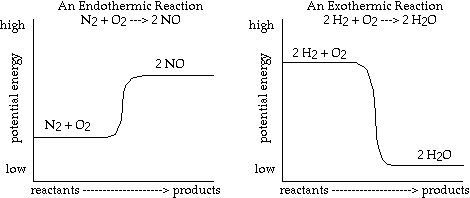
- In the diagram on the left the bonds of the NO molecules have more potential
energy than the N2
and O2
molecules. Running this reaction is analogous to compressing a spring. The
NO molecules are like the compressed spring in the sense that energy is stored
in the tensed spring.
- In the diagram on the right, it is analogous to the release of a compressed
spring - energy is released in the formation of water from hydrogen and oxygen.
- Reactions which are exothermic are most likely to be spontaneous, or at
least continue spontaneously once they have been started.
- The potential energy graphs above are simplistic views of the energies involved
in completing a reaction. In reality, the molecules usually need some extra
energy to initiate the reactions. The energy needed for a reaction to move
forward is called the Activation Energy and is abbreviated Ea.
A more realistic representation of the potential energy curves for the above
reactions is represented below:
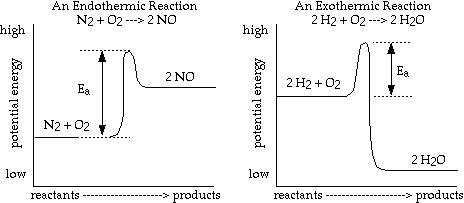
- Notice that the endothermic reaction needs a continual input of energy to
continue reacting. However, the reaction between H2
and O2
gives off so much energy that it can supply the left over reactants with the
activation energy needed to form water and complete the reaction. Once this
reaction is started with a spark or a flame it continues until there are no
more reactants.
- Exothermic reactions with a very low activation energy will occur spontaneously.
Exothermic reactions with a high activation energy may need some extra energy
to get started, and depending on the difference between the products and reactant
may need a continual input of energy to continue.