Bronsted-Lowry Definition
- Water is a polar molecule with the oxygen atom being somewhat negative.
Whenever a H+1
ion is dissolved in water, it is attracted to the negative oxygen. In fact,
it forms a new ion with water molecules called the hydronium ion: H3O+1
. It is therefore correct to write aqueous hydrogen ions as H+1
or H3O+1.
- To illustrate how HCl dissolves in water we could do either of the following:
- HCl(g)
---> H+1(aq)
+ Cl-1(aq)
or
- HCl(aq)
+ H2O(l)
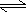 H3O+1(aq)
+ Cl-1(aq)
H3O+1(aq)
+ Cl-1(aq)
- According to Bronsted and Lowry you could think of a acid as a proton donor
(H+1
donor) and a base as a proton acceptor. Looking at the dissolution of HCl
in water from this perspective makes HCl the acid (H+1
donor) and water the base (H+1
acceptor). Because this is an equilibrium equation the hydronium ion can now
be a proton donor and the chloride ion can be a proton acceptor. The terms
used to describe the hydronium ion and chloride ion are conjugate acid and
conjugate base respectively.
- The general definition can be symbolized as:
| HA(aq)
|
+ |
H2O(l)
|
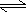
|
H3O+1(aq)
|
+ |
A-1(aq)
|
| acid |
|
base |
|
conjugate acid |
|
conjugate base |
- Using this expanded definition of acids and bases we can explain why NH3
is considered to be a base.
- NH3(aq)
+ H2O(l)
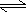 OH-1(aq)
+ NH4+1(aq)
OH-1(aq)
+ NH4+1(aq)
- Notice that in this case water acts as an acid, donating a proton to the
ammonia molecule which is the base. This shows that water can be an acid or
a base.
 H3O+1(aq)
+ Cl-1(aq)
H3O+1(aq)
+ Cl-1(aq)
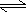
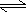 OH-1(aq)
+ NH4+1(aq)
OH-1(aq)
+ NH4+1(aq)