Dynamic Nature of Equilibrium
- Many systems around us attain some sort of equilibrium. You can
think of it as a kind of law of nature - that all systems seek equilibrium.
- Some everyday life examples of equilibrium.
- The population of some countries (not many) has reached an equilibrium between the number of deaths and births such that the number of people in the country remains constant.
- During passing time in the hallway there is an equilibrium. that is reached between the number of students at either end of the building. Some go from one end to another, but the number of students in either half remains constant.
- Perhaps your bank account always seems to have the same amount of money in it even though you continue to have a job and deposit money. You also withdraw money to spend on various things you want/need to buy.
- The amount of oxygen in your blood remains at a relatively constant level because as your body uses it for various chemical reactions you continue to breathe and take in oxygen.
- Some chemical equilibrium systems:
- Vapor pressure. We know that if we seal a liquid inside
a container, that some of the vapor will evaporate and begin to exert
a pressure on the walls of the container. As more of the water evaporates
there is a greater chance some of the vapor will recondense. Eventually
the pressure due to the vapor remains constant. However, the molecules
which are causing this pressure are not always the same molecules.
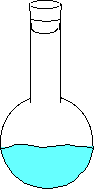
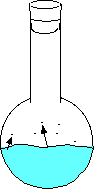
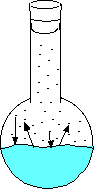
Here the water has just been added and no vapor has evaporated. Here the water begins to evaporate. The gaseous molecules are beginning to increase the pressure inside the flask. Eventually enough molecules are bouncing around such that the rate of evaporating molecules equals the rate of condensing molecules. This is when you can measure the equilibrium vapor pressure. - If you place a straw in a liquid, it may look like nothing is happening, but the pressure of the gas inside the straw is equal to the gas pressure from the atmosphere on the liquid outside of the straw. The opposing forces are equal preventing movement of the liquid in the straw.
- If you make a saturated solution of sodium chloride, such that there is a pile of undissolved solid on the bottom of the solution. An equilibrium is reached where the same number of ions dissolve into the solution as ions which become part of the solid again. The picture is much like that of the equilibrium state of vapor pressure mentioned above.
- The Hydrogen Spout demonstration came to equilibrium when the rate of molecules entering and leaving the porous cup became constant. See Molecular Dynamics Model of Diffusion. (diffusion-he/kr diffution)
- Osmosis of water entering and leaving a cell is a type of equilibrium. See Molecular Dynamics Model of Osmosis. (diffusion->osmotic pressure)
- Chemical reactions can also form an equilibrium. The example below is of Oxygen molecules breaking up to form single Oxygen atoms and reforming the diatomic molecules: O2 <---> 2 O See Molecular Dynamics Model of this chemical reaction. (condensed phases->Le Chetalier->starting point: add more O)
- Vapor pressure. We know that if we seal a liquid inside
a container, that some of the vapor will evaporate and begin to exert
a pressure on the walls of the container. As more of the water evaporates
there is a greater chance some of the vapor will recondense. Eventually
the pressure due to the vapor remains constant. However, the molecules
which are causing this pressure are not always the same molecules.
- Some everyday life examples of equilibrium.