- Because the reaction is: HC2H3O2(aq)
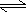 H+1(aq)
+ C2H3O2-1(aq)
H+1(aq)
+ C2H3O2-1(aq)
we know that for every acetic acid molecule that dissociates we will get one hydrogen ion and one acetate ion. This can be represented algebraicly in the following manner:Initial Concentrations Equilibrium Concentrations [HC2H3O2] = 3.0 M
[H+1] = 0.50 M
[C2H3O2-1] = 0.50 M[HC2H3O2] = 3.0 - x
[H+1] = 0.50 + x
[C2H3O2-1] = 0.50 +x - At equilibrium we know that
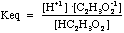 = 1.8x10-5
= 1.8x10-5 - Substituting the equilibrium concentrations above we get:
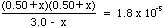
- Next set up a quadratic equation:
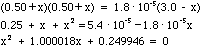
- Find possible solutions for x:
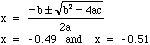
- Notice that only one solution for x makes physical sense: -0.49
- If we use -0.51 for x then we would end up with negative equilibrium concentrations, a physcial impossibility.
- Solving the equations above gives us equilibrium concentations of:
[HC2H3O2] = 3.49M
[H+1] = 0.01M
[C2H3O2-1] = 0.01M