Determining pH and Kw
- pH is a measure of how acidic or basic a substance is.
- It is a scale which ranges from 0 (most acidic) to 14 (most alkaline).
- The "pX" function of something stands for the "-log( X)",
so pH actually stands for
the -log([H+1]).
You should have seen this relationship in the serial dilution lab.
- Neutral water has a pH of 7. That implies that there must be some H+1
ions in pure water. Where might these ions come from?
- Water, in fact, ionizes in an equilibrium reaction as follows: H2O(l)
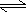 H+1(aq)
+ OH-1(aq)
H+1(aq)
+ OH-1(aq)
- We can determine the hydrogen ion concentration in water from
measuring its pH.
- Pure water has pH = 7. That means the [H+1]
= 1.0 x 10-7 M
- Because you get one OH-1
for each H+1
the [OH-1]
= 1.0 x 10-7 M
- Therefore Kw = [H+1][OH-1]
= 1.0 x 10-14 M
- By adding acids to water you are actually disturbing the equilibrium. As
more H+1
ions are dissolved the equilibrium shifts left and consumes hydroxide ions.
If a base is added, then the equilibrium will again shift to the left consuming
H+1.
That is how a base lowers the pH value. If the H+1
concentration decreases then the pH increases (becomes more alkaline).
- Practice calculations:
- What would be the pH of a solution with [H+1]
= 0.025 M?
- What would be the pH of a solution with [OH-1]
= 0.025 M?
- What would be the [H+1]
and [OH-1]
of a solution with pH = 4.55?
Click
here for more practice.
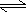 H+1(aq)
+ OH-1(aq)
H+1(aq)
+ OH-1(aq)