Solubility Product
- As you learned earlier this year some substances dissolve and others
don't - at least they don't seem to. In fact, everything dissolves at least
a little bit. Those substances which appear to not dissolve just dissolve
too little for us to observe with our eyes.
- One way of quantifying how well substances dissolve is to calculate the
equilibrium constant for the chemical equation which describes their dissolving.
- Let's use Silver Chloride for example:
AgCl(s)
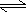 Ag+1(aq)
+ Cl-1(aq)
Ag+1(aq)
+ Cl-1(aq)
- The K value for describing the dissolving process is given
a special name: Ksp.
- The Ksp for the above reaction is: Ksp = [Ag+1][Cl-1].
- If the Ksp for a substance is large then it is quite soluble. If it
is very small then we would call the substance insoluble. The Ksp for
AgCl is 1.6 x 10-10 at 25°C, a very insoluble compound.
- Sample calculations:
- Calculate the concentration of silver ion at room temperature (25°C)
in a saturated solution of silver chloride. Click
here for answer.
- Calculate the concentration of fluoride ion for BaF2
if its Ksp is 2.4 x 10-5 at 25°C. Click
here for answer.
 Ag+1(aq)
+ Cl-1(aq)
Ag+1(aq)
+ Cl-1(aq)