Weak vs. Strong Acids and Bases
- The strength of an acid or base depends on its ability to dissolve
in water. The dissolving process can be represented by an equilibrium equation
in either of two forms:
- HA(aq)
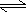 H+1(aq)
+ A-1(aq)
H+1(aq)
+ A-1(aq)
or
- HA(aq)
+ H2O(l)
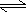 H3O+1(aq)
+ A-1(aq)
H3O+1(aq)
+ A-1(aq)
- HA(aq)
- The equilibrium constant for this equation is given the symbol Ka.
- So, in general
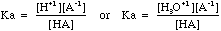
- The further the equilibrium lies to the right the stronger the acid and the higher the Ka value. See the list of Ka values for more detail.
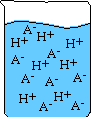
- The stronger the acid the more ions that are formed. See illustration below
of a strong and weak acid dissolved in solution:
Weak Acid Strong Acid 
- A good example of a weak acid is phenolphthalein: Click here to see how it works.