Scientific Notation/Units
- Scientific Notation
- Used when expressing very large or very small numbers.
- Eliminates ambiguous numbers of significant digits (more on this later)
- Is written in the form such that the first number is greater than or
equal to 1 and less than 10, and is multiplied by some factor of 10.
- 1.04 x 10^6 would be equal to 1,040,000
- 3.650 x 10^ -14 would be equal to 0.00000000000003650
- you will see different forms for scientific notation but all of
the following are equivalent
- 2.4 x
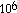 = 2.4 x 10^6 = 2.4 x 10+6 = 2.4 e+6
= 2.4 x 10^6 = 2.4 x 10+6 = 2.4 e+6
- 2.4 x
- Try this now on your calculator to see which form it uses to display scientific notation. You can see this by repeatedly multiplying or dividing some number by a very large number.
- To get some practice with converting numbers between "ordinary"
decimal notation and scientific notation go to:
http://janus.astro.umd.edu/astro/scinote/
- Units
- Prefixes are commonly used in expressing a unit of measurement.
Prefix Symbol Factor Meaning Prefix Symbol Factor Meaning yotta Y 1024 septillion yocto y 10-24 septillionth zetta Z 1021 sextilltion zepto z 10-21 sextillionth exa E 1018 quintillion atto a 10-18 quintillionth peta P 1015 quadrillion femto f 10-15 quadrillionth tera T 1012 trillion pico p 10-12 trillionth giga G 109 billion nano n 10-9 billionth mega M 106 million micro µ 10-6 millionth kilo k 103 thousand milli m 10-3 thousandth hecto h 102 hundred centi c 10-2 hundredth deca da 101 ten deci d 10-1 tenth You should memorize the ones in bold. We will use them often.
Go to: http://www.wordwizz.com/pages/10exp27.htm or http://micro.magnet.fsu.edu/primer/java/scienceopticsu/powersof10/ to get a sense of the difference between these powers of ten.- We will be using metric units for all our measurements
in this class so you should memorize the following:
- length will be measured in meters (m) or centimeters (cm)
- volume in centimeters (cm^3), milliliters (mL), or liters (L)
- mass in grams (g) or kilograms (kg) - Note: at no time will we be measuring the weight of any substance in this class, only its mass.