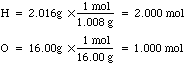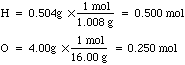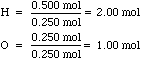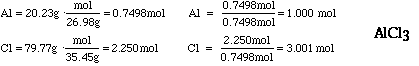Empircial Formulas
- An empirical formula states the simplest whole number ratio of
atoms in a substance.
- All ionic formulas are empirical (for example we write NaCl instead of Na2Cl2
or Na3Cl3)
and some molecular formulas are empirical.
- For example:
- Aluminum sulfide (ionic) is Al2S3
and is empirical.
- Water (molecular) is H2O
and is empirical.
- Benzene (molecular) is C6H6 which is not empirical.
Its empirical formula is CH.
- If we have a sample of a substance and can determine the mass of each element
present in the sample then we can determine its empirical formula.
- To understand how to do this, let's take water for example. In one mole
of water we have 2.016g of H and 16.00g of O. If we convert each of these
masses into moles we get the familiar ratio: 2.000 moles of H to 1.000 mole
of O. See calculation below:
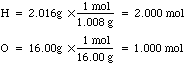
- If we only had one quarter of a mole of water then the H mass would be
0.504g and the O mass would be 4.00g. If we convert these masses to moles
then we get:
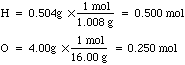
- By simplifying the mole ratio we still get a nice
whole number ratio, from which we can construct the correct empirical formula
- H2O:
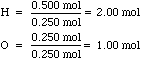
- If you had a sample of aluminum chloride that contained 20.23g of aluminum
and 79.77g of chlorine, what would be its empirical formula? Click
here to see answer below.
- Given a percent composition you should also be able to calculate the empirical
formula. If you know something is 27% carbon and 73% oxygen, just hypothesize
a theoretical sample of any arbitrary size and determine what the mass of
carbon and oxygen would be. For example, if you had a sample of the above
compound with a mass of 100g, then 27g would be carbon and 73g would be oxygen.
You can now start the empirical formula calculations.
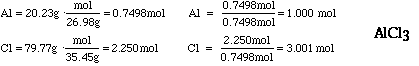
Jump back to the outline.