Counting Atoms and Molecules
- When doing reactions chemists need to count atoms and molecules. The problem of actually counting individual atoms and molecules comes from the fact that they are so small. Instead of physically counting each one we can measure the mass of a large group of them. If we know the mass of just one atom or molecule then we can figure out how many we have.
- Because we will always be dealing with groups of atoms and molecules it would be convenient to give a name to a certain number of them. Just as we can talk about groups of objects like a dozen (12) eggs or a gross (144) of pencils, we need a unit for a large number of atoms or molecules. This unit is the mole.
- 1 mole = 6.02 x 1023. This is also known as Avagadro's number, in honor of his contributions toward the development of atom counting experiments and theories.
- To give you some idea of how large a mole of items is think about the following:
- Spreading one mole of rice grains over the surface of the earth would create a layer one meter deep.
- If you had a mole of pennies and decided to give every person on Earth $1,000,000 a day, then it would take about 3000 years to spend all of your pennies.
- Even with today's high speed technology we still need to count by weighing. A computer counting 1 million atoms a second would take 20 years to count out one mole of atoms or molecules.
- Demo: Tray of moles.
- Weighing atoms
- So, it's too hard to count atoms but we can measure the mass of a large group and know how many we have. To do this we need to know the mass of an individual atom or the mass of a specific number of atoms.
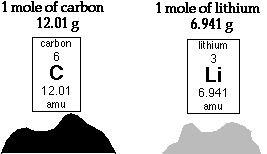
- The mass of one carbon-12 atom is very tiny - 1.99 x 10-23g.
- This mass is a bit cumbersome to deal with so a unit of mass was invented to make it easier to discuss the mass of atoms and molecules - the atomic mass unit (amu). It was defined such that the carbon-12 atom was exactly equal to 12.000 amu The mass of every other atom was then measured relative to this standard. If an atom weighed twice as much as a carbon-12 atom it had a mass of 24.000 amu
- So someone thought, "How many atoms of carbon-12 would there be in 12.000g of those atoms?". The answer is 6.02 x 1023 or 1.00 mol.
- Because 12.000g of carbon is an easily manageable amount of substance to use, the mole became our standard unit for counting the numbers of atoms or molecules.
- Every atomic mass on the periodic table has the unit amu, and if we have enough of an element measured out in grams to equal its atomic mass then we have 1.00 mol of that substance.
- Remember the term mole is just like the term dozen, it just represents a certain number of things. See the illustration bellow for a clarification of the relationship between atomic mass and the mole.
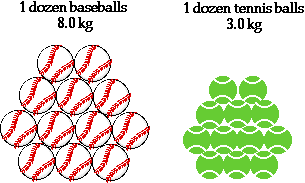
Notice the same number of baseballs and tennis balls can be referred to as a group (a dozen) but they can have different masses. The same is true for substances, except we refer to moles instead of dozens.