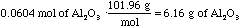Grams-Grams
- Grams-Grams
- When we are working with substances in the lab we use balances
to measure out how much substance to work with. So, we need to use the
reading from the balance to make similar predictions about the mass of
substances produced or needed to complete a chemical reaction.
- To do this, we need only do the appropriate conversions from grams to
moles and back again to utilize the MOLE RATIO we can derive from
the balanced chemical equation.
- Consider the following problem: Given the reaction between aluminum
and oxygen to produce aluminum oxide, what mass of aluminum oxide would
be produced if we started with 3.26g of aluminum?
- In order to solve this, we will need to use the chemical equation to
tell us the relationship between the moles of Al and the moles of Al2O3.
However, our Al quantity is in grams. First we must convert the grams
to moles.

- Then, set up a mole ratio:
| 0.121 |
|
|
|
x |
| 4 Al |
+ |
3 O2
|
---> |
2 Al2O3
|

- Then, convert the moles to grams.
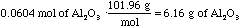
- Now we have our answer: If we start with 3.26g of Al, we should expect
to produce 6.16g of Al2O3.