Molecular Formulas
- In order to calculate the molecular formula for molecular substances you do the same procedure as finding the empirical formula and add one more step that requires one more piece of information - the molar mass of the molecular formula.
- To understand how the last step works consider the relationship between
the empirical and molecular formula of benzene:
Empirical Formula
CHMolar Mass
13.02gMolecular Formula
C6H6Molar Mass
78.12g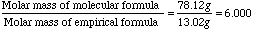
- Notice that if you divide the molecular formula mass by the empirical formula mass you get a factor by which you should multiply each atom of the empirical formula. So CH becomes C6H6.
- Try the following. If a compound with the empirical formula CH2O
was found to have a molar mass of 90g, what would the molecular formula be?