Soap
- There are two reasons why water doesn't clean well by itself. Both
of these reasons have to do with water's high polarity.
- The strong surface tension of water prevents it from getting
into small spaces.
- Most dirt is non-polar so it doesn't dissolve well in polar water.
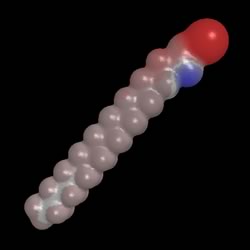
- Sodium stearate is a common soap which solves both of the problems described
above.
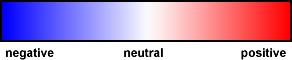
Notice that one end is polar and the other end is non-polar.
This make sodium stearate a kind of schizophrenic molecule. It can dissolve
in both polar and non-polar substances.
- To see how this breaks up water's surface tension: Breaking
up Surface Tension.
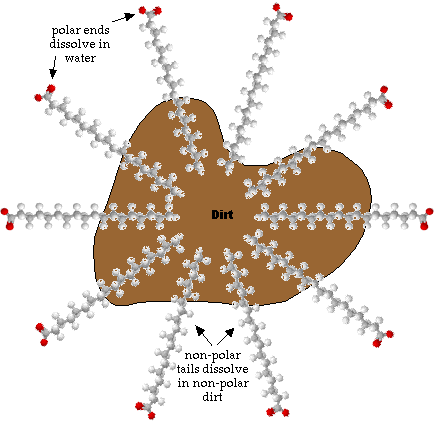
- When soaps are dissolved in water the molecules arrange themselves into
groups called micels. The non-polar ends will congregate together while the
polar ends will interact with the water. Because dirt is mostly non-polar
the dirt gets dissolved into the center of the micelle.