Solubility Curves
- Qualitative Description of Concentration
- A solution can have more or less solute dissolved in the solvent.
This can be described in general terms as either unsaturated, saturated,
or supersaturated.
- Unsaturated: More solute can be dissolved in the
solvent.
- Saturated: The maximum amount of solute is dissolved in the
solvent.
- Supersaturated: More solute is dissolved than can normally
be dissolved in the solvent
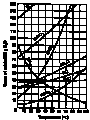
- Several factors can affect how well something will dissolve.
- Temperature
- See the solubility curves of several substances by clicking
on the image to the right.
- Notice the pattern for most of the substances? What do these
substances have in common?
- What is different about the one substance listed that doesn't
follow the same pattern?
- Pressure (only for gasses): The higher the pressure the more gas
can be dissolved in the solvent. Think abouut what happens when you
reduce the pressure inside a bottle of soda when you open it.
- Type of solvent and solute. "Like dissolves like."
