Mendeleev's First Periodic Table
- The first periodic table was arranged by Dimitri Mendeleev in 1869.

- He was a professor of Chemistry. at the University of St. Petersburg
in Russia and was confronted with the problem of how to teach about the
various elements known at that time. He decided to organize the elements by studying properties of the elements. He looked at things like:
- Density
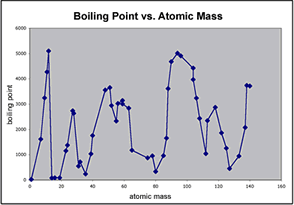
- Melting point
- Chemical Formula
- He noticed that if you arranged the elements in order from lowest atomic mass to highest that various properties would repeat "periodically".

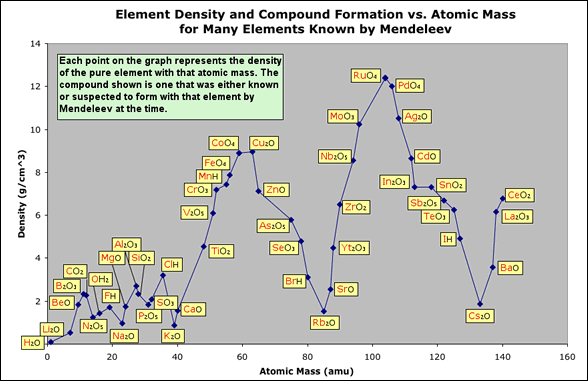
- He also saw that the compounds formed by those elements also changed periodically forming the following pattern with R representing some element of interest : R2O, RO, R2O3, RO2, R2O5, RO3, RH. Below is a density graph like the one above with the addition of a compound formed by the element at each data point. Click on the graph to view it full screen.
- Mendeleev arranged a table of elements with rows and columns based on the patterns he saw in the graphs above. What patterns can you find?
- Using the data in the graphs above, Mendeleev studied the element patterns and found gaps where it seemed that an element was missing. He predicted that new elements would be discovered to fill in the missing parts of his table. In the table above he predicted three new elements that would be discovered with atomic mass less than 80. Between which elements would you predict another element should exist.
- What properties would you predict for these new elements (they are listed from lowest atomic mass to highest):
Atomic Mass (amu) Formula Density (g/cm3) predicted actual predicted actual predicted actual Ekaboron (Scandium) 



not predicted by Mendeleev 
Ekaaluminum (Gallium) 





Ekasilicon (Germanium) 





- So, Mendeleev created a table with rows and columns. Elements in the same column would have the same chemical properties (for compounds in a similar pattern) and physical properties would repeat periodically as you changed rows. This organization of the elements is what allowed him to make the above predictions about missing elements and their properties.
- Go to: http://www.periodic.lanl.gov/mendeleev.htm to see a version of Mendeleev's first table.
- He was a professor of Chemistry. at the University of St. Petersburg
in Russia and was confronted with the problem of how to teach about the
various elements known at that time. He decided to organize the elements by studying properties of the elements. He looked at things like: