The Equilibrium Constant
- As you have seen from the computer lab simulations. The ratio of products to reactants is a constant. Let's study this in more detail. The equilibrium constant will be represented by the symbol Keq.
- There are two classifications of reactions that are necessary to write proper
equilibrium constants: homogeneous and heterogeneous equilibria
- Homogeneous equilibria
- Heterogeneous equilibria
- pure liquids or solids don't have any affect on the final result of Keq, so they are not included in its calculation if these types of substances are present in the reaction.
- Ex. 1: PCl5(s)
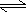 PCl3(l)
+ Cl2(g)
PCl3(l)
+ Cl2(g)
Keq = [Cl2] - Ex. 2: 2 KClO3(s)
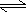 2 KCl(s)
+ 3 O2(g)
2 KCl(s)
+ 3 O2(g)
Keq = [O2]3 - Ex. 3: NH4NO3(s)
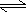 N2O(g)
+ 2 H2O(g)
N2O(g)
+ 2 H2O(g)
Keq =
- Calculating the equilibrium constant
- Consider the following reaction: HC2H3O2(aq)
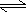 H+1(aq)
+ C2H3O2-1(aq)
H+1(aq)
+ C2H3O2-1(aq)
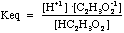 Try starting with
different amounts of reactants and products and push the equilibrate button
to see what the equilibrium conditions will be.
Try starting with
different amounts of reactants and products and push the equilibrate button
to see what the equilibrium conditions will be.
- Type in several different starting concentrations above.
- What do you notice about the value for Keq?
- If you put in a small number for the acid concentration, a large number for one of the ion concentrations, and then put in zero for the other ion concentration. What happens when the system comes to equilibrium? Why?
- After putting in pure acetic acid and allowing the system to equilibrate you measure the hydrogen ion concentration to be 0.150 M. What would the equilibrium concentrations be for [C2H3O2-1] and [HC2H3O2]? Click here for answer.
- Given the following equation: H2(g)
+ I2(g)
 2 HI(g)
Keq=20
2 HI(g)
Keq=20
If a flask contains HI at a pressure of 200 mmHg, H2 at a pressure of 10 mmHg, and I2 at a pressure of 4 mmHg, will the system produce more reactants or more products to reach equilibrium? Click here for answer. - Now try a calculation using the acetic acid dissociation described
above. Assume Keq=1.8x10-5 and the initial conditions to be the
following: [HC2H3O2]
= 3.0M; [H+1]
= 0.50M; [C2H3O2-1]
= 0.50M. What would be the equilibrium concentration of all
products and reactants? Click
here for answer.
- Consider the following reaction: HC2H3O2(aq)